How to make a Data Request (For External Collaborators)
Overview
These data request forms are for data and/or specimen requests made by Non-LIMBIC-CENC affiliated investigators, researchers, and staff, and their invited collaborators. These forms can be found under the “How to make a data request” tab on the left side of the page.
If you are associated with LIMBIC-CENC, please Login to the website and submit your request through the Data Request portal in Consortium Operations page.
Download and complete the preliminary project proposal form to describe your proposed research and requested use of existing LIMBIC-CENC data and/or biospecimen. The following flowchart provides an overview of the data and/or specimen request and review process, and a detailed description of the process can be found below:
Preliminary Data Specimen Request Proposal Form
Full Data Specimen Request Proposal Form
Data Dictionary Portal
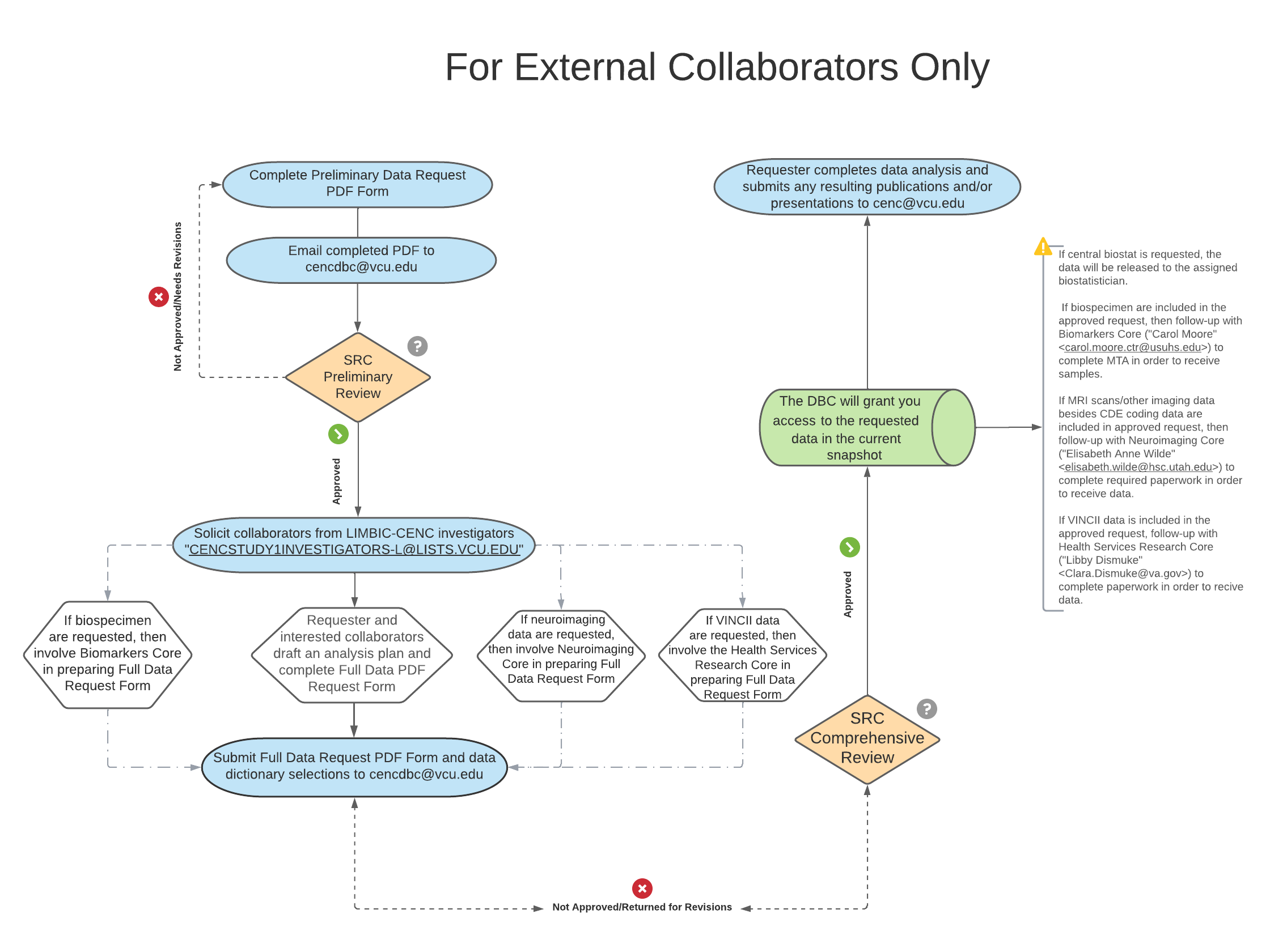
The data request process consists of two forms: (1) a preliminary and (2) a full proposal. These forms are used for any data and/or specimen requests made by any LIMBIC-CENC affiliated investigators, researchers, and staff, and their invited collaborators. These forms are not intended for requests from non-LIMBIC-CENC investigators and/or public use. Please see the publication and data request policy (coming soon) for a detailed definition of entities covered.
Part A: Complete the preliminary proposal to describe your proposed research and requested use of existing LIMBIC-CENC data and/or biospecimen. A complete application includes:
- The completed preliminary proposal form, with all fields filled out.
- For any collaborators not directly affiliated with the LIMBIC-CENC, a biosketch is required.
- The preliminary proposal must be approved by the Scientific Review Committee before proceeding to Part B.
Part B: Complete the full proposal once you have finalized your list of collaborators. A complete application includes:
- The completed full proposal with the final list of collaborators and fully developed analysis plan (no longer than 6 pages, excluding tables and graphs).
- Selected variables from data dictionary
- Separate PCE Variable selection, if applicable
- Separate Medication Variable selection, if applicable
- For any collaborators not directly affiliated with the LIMBIC-CENC, a biosketch is required.
- IRB approval letter, if applicable
Any LIMBIC-CENC investigator may initiate a proposal by emailing a completed preliminary proposal to cencdbc@vcu.edu; indicate “Data Request” in the subject line. The Scientific Review Committee will first perform a screening/preliminary assessment for scientific value, potential duplication or conflict with completed or pending projects. For approved preliminary proposals involving data from the Multicenter Observational Study (LIMBIC-CENC Prospective Longitudinal Study, i.e., CENC Study 1), all Prospective Longitudinal Study investigators must also be notified of the research proposal to allow for identification of additional potential collaborators. Notification occurs via CENCSTUDY1INVESTIGATORS-L@LISTS.VCU.EDU with a 10 business day response period. Interested collaborators should express what value they would add to the project and the project PI has final decision on who to include as collaborator and/or coauthor. The documentation for this should be included in the full proposal after the notification period is complete. For requests for use of LIMBIC-CENC resources for new funding proposals, solicitation of potential collaborators is not required; however a justification is required in the comments field for why collaborators were not pursued.
Once the final list of collaborators is generated, the project PI will work with interested collaborators to complte the full proposal. The full proposal, along with the data dictionary selections, will be submitted to the Scientific Review Committee for a much more thorough review, assessing the above as well as general suitability of data and/or specimens requested, and confirm approval by relevant core directors (i.e., Biomarkers Core for biospecimen requests, Neuroimaging Core for specialized imaging data). The LIMBIC-CENC Data and Biostatistics Core will then further vet the details of the request if support from the LIMBIC-CENC Data and Biostatistics Core is requested. Once the feasibility of the project is confirmed, the LIMBIC-CENC Research Committee will give final approval, and an analyst will be assigned to pull the data. During this evaluation process, the Scientific Research Committee may communicate back to the project PI to seek further details, clarifications, and/or recommend changes. Please note that all data requests will be based on the number of participants “graduated to follow-up” (i.e., completed PCE mapping, portions of neuropsychological tests battery, DRRI-2) unless otherwise requested.
Once your full proposal has been submitted, the LIMBIC-CENC Research Committee reviews and vote on the proposal. The requestor will be notified shortly thereafter of the final decision.
Please note: any communications about a data request should be CC’d to CENCDBC@vcu.edu . Publications and/or presentations of research that use LIMBIC-CENC data or biologic specimens must be submitted to the LIMBIC-CENC Publications Committee at cenc@vcu.edu.


